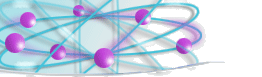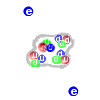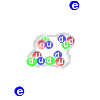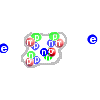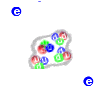
An atom of helium |
Protons and neutrons
group together to form atomic nuclei, and these are surrounded by
electrons spinning around them.
In an atom, the number of electrons is equal to that of the protons.
So, iron has 26 protons and 26 electrons.
This structure, formed by the nucleus and the electrons spinning around
it, is an atom, and all the particles in it are in continuous movement.
In the animation the
sizes and distances are not to scale. If the nucleus of an atom was the
size seen in the animation, we should draw the electrons a kilometre
away! Actually the nucleus is 10 billion times smaller than that shown
in the animation.
|
The simplest atom is that of
hydrogen. Its nucleus is a simple proton, with a single electron
spinning around it. The next is helium with two protons and two
neutrons in the nucleus, and two electrons around it. And so on up to
the largest atoms which can have as many as 100 protons and a little
more than 150 neutrons in the nucleus.
Next
|